Very common 10 or more. 1-16 of 428 results for benzoyl peroxide 10 Skip to main search results Eligible for free delivery.
Specialty Benzoyl Peroxide Bp 5 Bp 10
It also has a mild drying effect which allows excess oils and dirt to be easily washed away from the skin.

Benzoyl peroxide 10 lotion. Peeling application site erythema. This powerful solution dries up pimples while keeping the skins moisture intact. Benzoyl peroxide is an amazing medication for acne treatment and there are generally two ways to use the product in a wash form or a leave-on gel or cream.
Benzoyl peroxide is a well-known ingredient for fighting acneAvailable in over-the-counter OTC gels cleansers and spot treatments this ingredient comes in different concentrations for mild. Compounding powder topical bar topical cream topical foam topical gel topical kit topical liquid topical lotion topical pad topical soap. Simply apply at night before bed or in the morning before you put on.
Generally speaking benzoyl peroxide can be a potentially-irritating ingredient for those with sensitive skin but the CeraVe Acne Foaming Cream Cleanser combines it with plenty of soothing and hydrating ingredients to mitigate the likelihood of irritation. 42 out of 5 stars based on 5 reviews 5 ratings Current Price 538 5. The lightweight lotion contains 5 percent benzoyl peroxide to kill off acne-causing bacteria plus moisturizing squalane to leave your skin soft and hydratednot over-stripped.
Applies to benzoyl peroxide topical. It may take several weeks to see the full effects. Oxy-10 for the skin is used to treat acne.
Benzoyl peroxide has three actions - it kills germs bacteria it reduces inflammation and it helps to unplug blocked pores. Clean affected part before use. This medicated gel treatment contains 10 benzoyl peroxide to help treat acne and prevent future breakouts.
Benzoyl Peroxide 10 Acne Treatment Gel - Pimple Cream Acne Spot Treatment - Fight Cystic Acne Back Acne Body Acne - by Claridad - Paraben Cruelty Free 44 out of 5 stars 389 2097 20. An impressive 10 of Jan Marinis acne-fighting lotion is benzoyl peroxide. Benzoyl peroxide may bleach hair or fabrics.
If you see benzoyl peroxide gel or white marks on your skin after you put it on you may be using too much. 96 496Fl Oz 649 649. 7 Mary Kay Acne Treatment Gel 5 Benzoyl Peroxide.
Per Tube Pack 2 Total 3 oz. Benoxyl Benzac AC Benzac W Benzagel Brevoxyl Desquam Fostex Lavoclen-4 Lavoclen-8 Neobenz Micro Persa-Gel Triaz. Not all brands are listed on this leaflet.
Untuk mengatasi jerawat pada orang dewasa dan anak-anak berusia di atas 12 tahun oleskan gel yang mengandung 2510 benzoyl peroxide pada jerawat 12 kali sehari. V55 MAX Double Strength Salicylic Acid Cream for Spots Blackheads Milia Blemishes Problem Skin Suitable and Safe for Those Prone to Acne - Paraben and Cruelty Free - 50ML. This material is provided for educational purposes only and is not intended for medical advice diagnosis or treatment.
It comes in different brand names and strengths - there is a 25 4 5 and 10 strength. Dryness pruritus contact sensitization reactions. Both PanOxyl products are dermatologist-recommended benzoyl peroxide face washes available in 4 and 10 formulations as we believe a wash is the best way to use this powerful.
Dosis dan Aturan Pakai Benzoyl Peroxide. Benzoyl peroxide has an antibacterial effect. What To Look For In a Benzoyl Peroxide Product The concentration of benzoyl peroxide.
Benzoyl Peroxide Gel Common BrandS. Acne Free Terminator 10 Acne Spot Treatment with Benzoyl Peroxide 10 Maximum Strength Acne Cream Treatment 1 Ounce - Pack Of 1 44 out of 5 stars 7666 496 4. Data sources include IBM Watson Micromedex updated 2 Feb 2021 Cerner Multum updated 3 Feb 2021 ASHP updated 29 Jan 2021.
Gently rub the cleanser into the skin for 10-20 seconds. Its a daily treatment that deeply penetrates pores to reduce the severity of acne blemishes giving you the confidence you need to take on any day feeling your best. You can buy benzoyl peroxide without a prescription at a pharmacy.
Use as you have been told even if your signs get better. Make sure to dry well. Common 1 to 10.
It even works as a preventative measure to keep future blemishes from forming. If this occurs flush the area with plenty of water. Cara Menggunakan Benzoyl Peroxide dengan Benar.
There are many brands and forms of benzoyl peroxide available. If using cleansers containing benzoyl peroxide wet the affected area. Use carefully and avoid contact with hair clothing and furnishings.
Benzoyl Peroxide 10 Generic for Persa Gel 10 Maximum Strength Acne Medication Gel for Treatment and Prevention of Acne Pimples Acne Blemishes Blackheads or Whiteheads. Product Title Rugby Acne Medication Benzoyl Peroxide Lotion 10 1.
It is a strong oxidizing and bleaching agent. Likewise catalase has one of the highest turnover numbers of all enzymes.
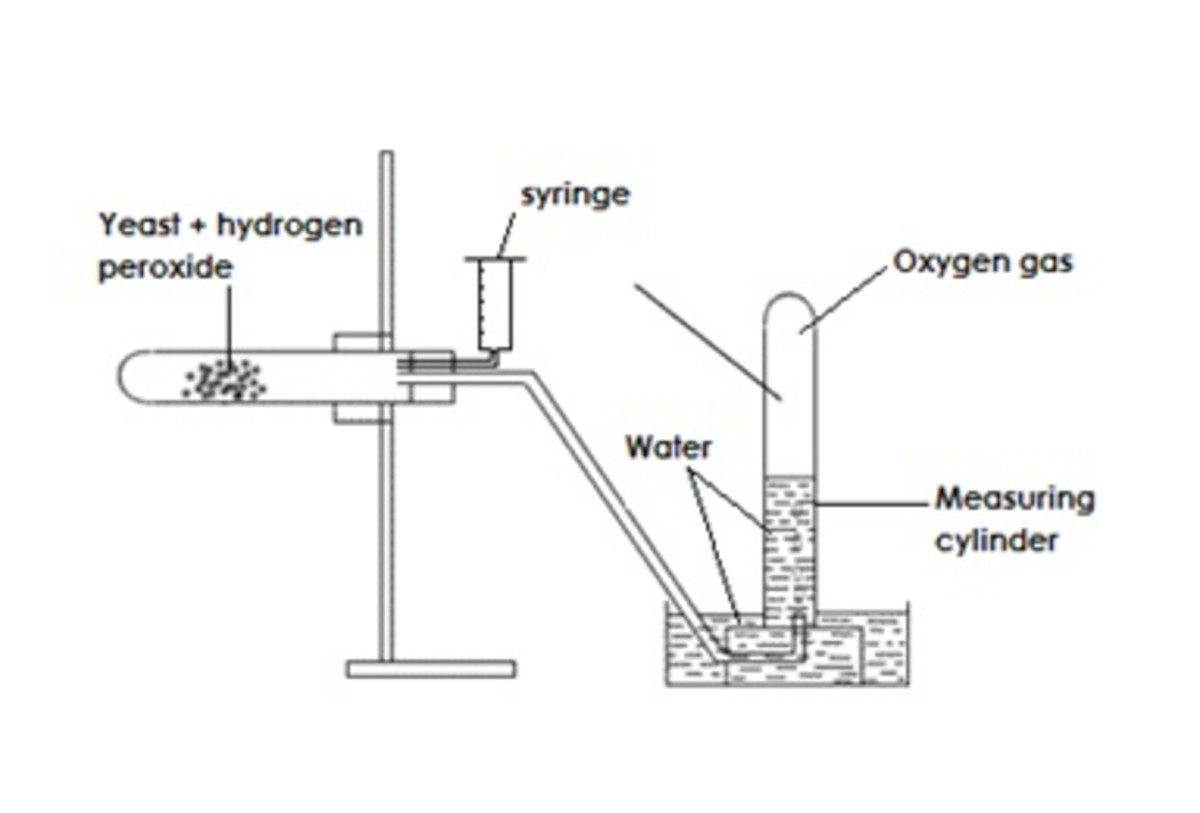 Effect Of Substrate Concentration On The Rate Of Activity Of Catalase Owlcation Education
Effect Of Substrate Concentration On The Rate Of Activity Of Catalase Owlcation Education
This is where catalase works by converting peroxide into heathy water and oxygen compounds.
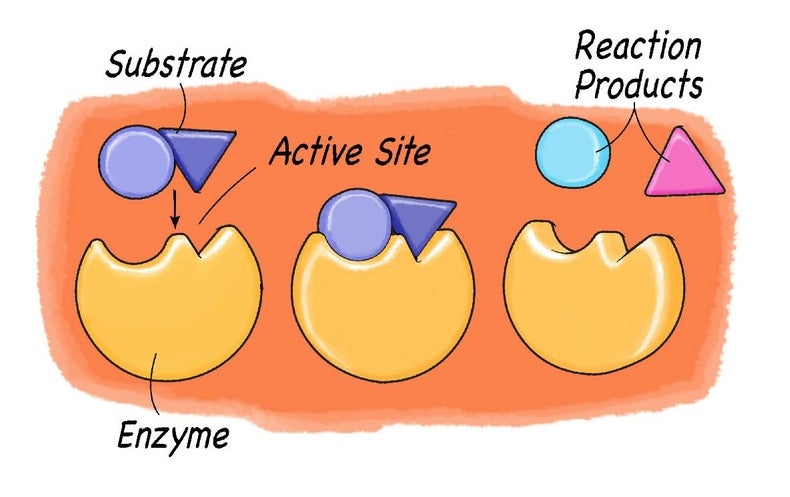
Catalase and hydrogen peroxide. 9495 In catalase the enzymatic reaction is the disproportionation of hydrogen peroxide Reaction 582 and the function of the enzyme appears to be prevention of any buildup of that potentially dangerous oxidant see the discussion of dioxygen toxicity in Section III. I seek to find the speed of effect between catalase and hydrogen peroxide. Catalase is a common enzyme found in nearly all living organisms exposed to oxygen that catalyzes the decomposition of hydrogen peroxide to water and oxygen.
Description of the Enzymes. This investigation looks at the rate of oxygen production by the catalase in pureed potato as the concentration of hydrogen peroxide varies. Oxidative stress is hypothesized to play a role in the development of many chronic or late-onset diseases such as diabetes asthma Alzheimers disease systemic lupus erythematosus rheumatoid arthritis and cancers.
However when there is a drop in catalase levels hydrogen peroxide cannot be decomposed. One catalase molecule can convert millions of hydrogen peroxide molecules to water and oxygen each second. This experiment looked at how substrate concentration can affect enzyme activity.
Catalase performs its rapid destruction of hydrogen peroxide in two steps. Catalase is a common enzyme found in nearly all living organisms exposed to oxygen which catalyzes the decomposition of hydrogen peroxide to water and oxygen. Hydrogen peroxide is produced naturally in human body.
Catalase is an important enzyme that protects cells from oxidative damage which hydrogen peroxide can cause. Catalase acts as the catalyzing enzyme in the decomposition of hydrogen peroxide. Hydrogen peroxide is the toxic by-product of respiration and would cause cell damage if it were not quickly removed.
The first step involves the catalase removing and binding one oxygen atom and releasing the rest of the hydrogen peroxide molecule as water. These observations have served to strengthe. Catalase breaks down and destroys hydrogen peroxide in two steps.
It is a very important enzyme in protecting the cell from oxidative damage by reactive oxygen species. Catalase converts the reactive oxygen species hydrogen peroxide to water and oxygen and thereby mitigates the toxic effects of hydrogen peroxide. Catalase decomposes or breaks down hydrogen peroxide into water and oxygen.
Remember H2O2 hydrogen peroxide is a bleaching agent whether you make it in your cells or buy it in a brown bottle from the pharmacy. Hydrogen peroxide is harmful and must be removed as soon as it is produced in the cell. Catalase is a tetramer of four polypeptide chains each.
The oxygen produced in 30 seconds is collected over water. It is an incredibly efficient enzyme where one catalase molecule can convert millions of hydrogen peroxide molecules each second. Hydrogen peroxide is produced during aerobic respiration the process by which cells generate usable energy by breaking down glucose and oxygen.
Genetic deficiencies of glucose-6-phosphate dehydrogenase G6PD and NADPH predispose affected erythrocytes to destruction from peroxides. This reaction is recognized as catabolic reaction as the hydrogen peroxide molecule is broken down into air and water which can be relatively smaller. One oxygen atom is extracted and attached to the iron atom and the rest is released as harmless water.
Catalase is an enzyme that breaks down hydrogen peroxide into water and gaseous oxygen. Conversely genetic deficiencies of catalase do not predispose affected erythrocytes to peroxide-induced destruction. Found extensively in organisms that live in the presence of oxygen catalase prevents the accumulation of and protects cellular organelles and tissues from damage by peroxide which is continuously produced by numerous metabolic reactions.
Catalase and Hydrogen Peroxide Catalases main job is to remove hydrogen peroxide H 2 O 2 by converting it into water and oxygen 1. Catalase an enzyme that brings about catalyzes the reaction by which hydrogen peroxide is decomposed to water and oxygen. The second step is the catalase breaking down another hydrogen peroxide molecule by releasing oxygen gas and water.
Then a second hydrogen peroxide molecule binds. Cells produce the enzyme catalase which breaks hydrogen peroxide down into two harmless substances water and oxygen. As hydrogen peroxide is active and harmful to cells and tissues of organisms its decomposition therefore needs to be speeded up greatly in order to prevent it from intoxication in the cell.
This enzyme like many others aids in the decomposition of one substance into another. Its turnover number can depend on 600 000 whereby one molecule of enzyme catalase can catalyse the decomposition of 600 000 molecules of hydrogen peroxide to oxygen and drinking water at bodys temperature. Digestive enzymes such as Catalase are healthy proteins molecules which have been found in living cells.
They can be used to improve specific reactions in the cellular material. GSH appears to be the primary antioxidant for protection against hydrogen peroxide since mutants lacking GSH gsh1 or glutathione reductase glr1 are sensitive whereas strains lacking catalase A cta1 or catalase T ctt1 are unaffected in resistance to this oxidant. The research outlined in this paper investigated this property of catalase and verified whether or not this enzymesubstrate relationship follows the Michaelis-Menten relationship.
Sometimes catalase also uses hydrogen peroxide to oxidise contaminants including Phenols Formic Acidity Formaldehyde and Alcohols. Catalase and peroxidase are heme enzymes that catalyze reactions of hydrogen peroxide. Catalase breaks down hydrogen peroxide into water and oxygen.
Reaction of catalase with hydrogen peroxide AIM. Nearly all living things possess catalase including us. Cells make the enzyme catalase to remove hydrogen peroxide.
Getting rid of peroxide is the second step to completely neutralizing the original superoxide grenade. First a molecule of hydrogen peroxide binds and is broken apart.