Thus most of the d block elements are transition metals. Very hard usually shiny ductile and malleable.

Elements that have paired d-suborbital electrons are diamagnetic.
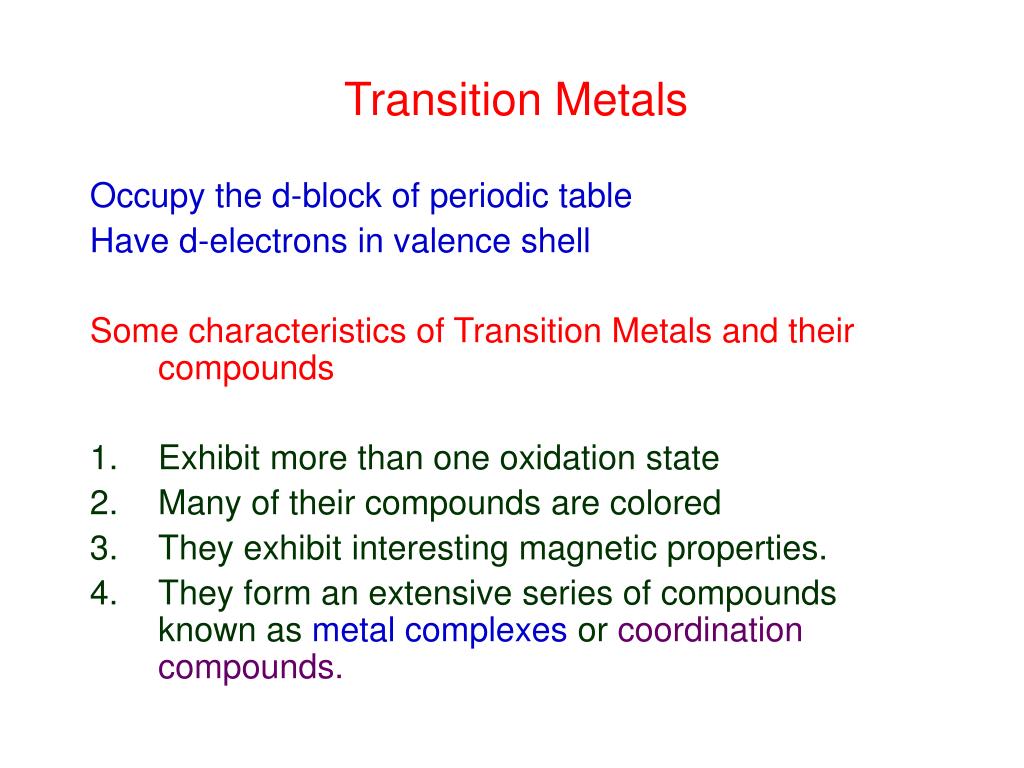
Chemical properties of transition metals. Much of these properties are brought about by the fact that the transition metals can form variable oxidation states. Transition elements that have one or more unpaired electrons in the d-suborbital will be paramagnetic. The document has moved here.
They are generally good conductors of heat and electricity and tend to crystallize in BCC body-centred cubic CCP cubic close-packed or HCP hexagonally close-packed structures. For example the lanthanides all form stable 3 aqueous cations. While there are radioisotopes of other elements all of the actinides are radioactive.
Are hard and have high densities. It is useful at the beginning to identify the physical and chemical properties of transition elements which differ from main group elements s-block. Forming compounds with variable oxidation states.
They have high melting points and densities and are strong and hard. A large range of complex ions in various oxidation states colored complexes and catalytic properties either as the element or as ions or both. In this paper we present density functional theory DFT investigations of the physical chemical and electronic structure properties of several close-packed surfaces of early transition metal carbides including v-Mo 2 C 0 0 0 1 and the 1 1 1 surfaces of TiC VC NbC and TaC.
This describes groups 3 through 12 on the periodic table although the f-block elements lanthanides and actinides below the main body of the periodic table are also transition metals. They are less reactive than alkali metals such as sodium they form coloured ions of different charges some are very. The elements of groups 411 are generally recognized as transition metals justified by their typical chemistry ie.
Lithium burns slowly with a red flame and liberates white fumes which become a white solid at the end of the reaction. These orbitals are buried inside the atom and are shielded from the atoms environment by the 4d and 5p electrons. Nihonium Nh flerovium Fl moscovium Mc and livermorium Lv are considered to possibly belong to the post-transition metal family though it is yet to be confirmed due to some unknown properties of the elements.
The transition metals have some characteristic chemical properties including. All transition elements exhibit similar properties because of the identical electronic configuration of their peripheral shell. Polonium is sometimes also included in the list of post-transition metals.
Properties of transition elements include. Properties of the Transition Elements. Have large chargeradius ratio.
The basic metals are similar to transition metals but tend to be softer and to hint at nonmetallic properties. In these elements at least the stable cations they form should have unpaired d electrons. A paramagnetic atom is attracted by a magnetic field while a diamagnetic atom is not attracted by the magnetic field.
According to the IUPAC a transition metal is any element with a partially filled d electron sub-shell. This creates an effective shield between the nucleus and the outer 4s shell. Lithium burns slowly with a red flame and liberates white fumes which become a white solid at the end of the reaction.
This happens as each additional electron enters the penultimate 3d shell. Chemical properties of transition metals. The elements 113-116 on the periodic table ie.
They form coloured compounds and act as catalysts. Transition metal any of various chemical elements that have valence electronsie electrons that can participate in the formation of chemical bondsin two shells instead of only one. The reddish-brown bromine vapour is decolourised.
Form compounds which are often paramagnetic. Transition metals are chemical elements having atoms with unpaired d electrons. The transition elements are metals.
Have high melting and boiling points. A strong tendency to form complexes. The peripheral shell configuration of these elements is ns 2.
Know that transition metals can be paramagnetic or diamagnetic. Transition metals demonstrate a wide range of chemical behaviors. The transition metals have the following chemical properties in common.
The chemistry of the lanthanides differs from main group elements and transition metals because of the nature of the 4f orbitals. The transition metals exhibit typical metallic properties such as malleability ductility high tensile strength and metallic lustre. In their pure state all of these elements tend to have a shiny metallic appearance.
As can be seen from their reduction potentials Table P1 some transition metals are strong reducing agents whereas others have very low reactivity.